An article by Julien Boissat
Last month, I attended a training session for SOLIDWORKS Reseller Technical Support teams. I was with my colleagues Henri and Andreas; Henri mentioned that he had been challenged to participate in the “ice bucket challenge” and showed us the video of the friend who challenged him. This person was standing with a bucket of water and a bag of ice cubes by his side. After his speech, he tore the bag, poured the ice cubes into the water; he quickly mixed and poured the mixture over it.
There is one thing we all immediately agreed on: the water certainly hadn’t had time to get really cold.
It is very convenient in this case to show that we really put ice cubes in the water !! To my knowledge, tap water does not cool instantly when you put ice cubes in it. You have to wait a while. This is a lesson I learned by putting ice cubes in my pastis, I mean in my soda (of course – always drink in moderation
).
So how long do you have to wait for the water to get really cold? To answer this question, any normally constituted person falls into one of these three categories:
But I’m not like everyone else, so I’m going to do a simulation. I know modeling a bucket of water with ice cubes in SOLIDWORKS Flow Simulation will be nowhere near as fast as performing the test in real life, but that doesn’t matter.
Likewise, SOLIDWORKS Flow Simulation is not really intended for doing this kind of simulation. It does a lot of things very well, but if you want to simulate melting ice cubes on the surface of water while everything is in motion, you’re going to have to compromise.
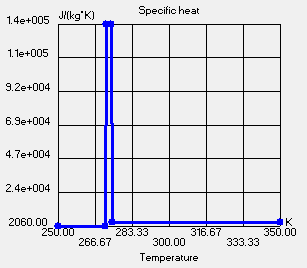
So, I grabbed a bucket of 3D ContentCentral (thanks Jesse Mathey). I added 60 ice cubes of 2.5 cm3 each, a lid (to create an internal space of 7 liters) and defined my projects.
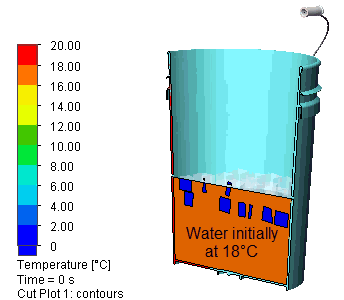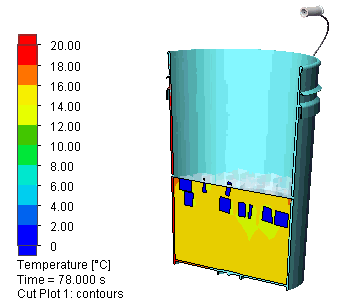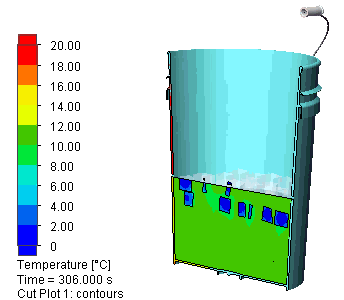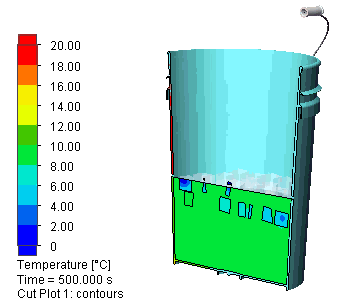
Here is what it looks like (half model, hidden cover):
I let the simulation run for 500s (about 8 minutes). Here is a view of the evolution of the temperature distribution:
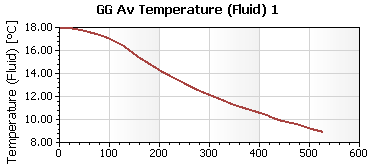
In the graph below you can see the average water temperature as a function of time:
Ultimately with all the guesswork we did and with roughly 450g of ice in that water it takes about 7 minutes to bring it back to 10 ° C which should feel quite cold.
Therefore, challengers of the Ice Water Bucket Challenge, take your time, leave the water alone, and let the ice cubes melt. Patience is a virtue. And to all viewers who suspect that there is cheating in the air when they don’t see giant ice cubes, beware, you could be wrong …
Next month we will study the cooling of beer bottles in a stream of cold water. I think I’ll just do a physical test for a change
.